Ibogaine is a naturally-occurring psychoactive compound frequently extracted from the root bark of Tabernanthe iboga, the plant source of ibogaine, which has its origins in the Bwiti cult of Gabon, a Central African religious group (1). In Gabon, the government has declared iboga as “a national treasure,” but in the U.S., ibogaine is listed as a Schedule I drug, meaning it is considered to have high abuse potential and no medical value. In other countries such as New Zealand, Mexico, the Bahamas, Canada, Australia, Spain, Brazil, Costa Rica, and South Africa, ibogaine treatment is currently used in clinical and medical contexts for treating substance use disorders (SUD). In these contexts, the ibogaine is often derived via semi-synthesis from a more widely available alternative African plant called Voacanga africana.
How ibogaine works in treating addiction is not yet fully understood. It is known to possess multiple mechanisms of action that can simultaneously alleviate the acute symptoms of opioid withdrawal, reduce opioid use and cravings for extended periods or permanently, engender novel insights about the psychological origins of one’s addiction, and improve mood. Human anecdotal reports and case studies have also indicated that ibogaine can help reduce cravings to a variety of other highly addictive drugs such as cocaine, amphetamines, and alcohol (2). Additionally, iboga has a host of medical benefits including antifungal and anti-parasitic properties, and is being investigated as a treatment for Parkinson’s disease due to its ability to regenerate dopamine cells in the brain.
Studying Ibogaine
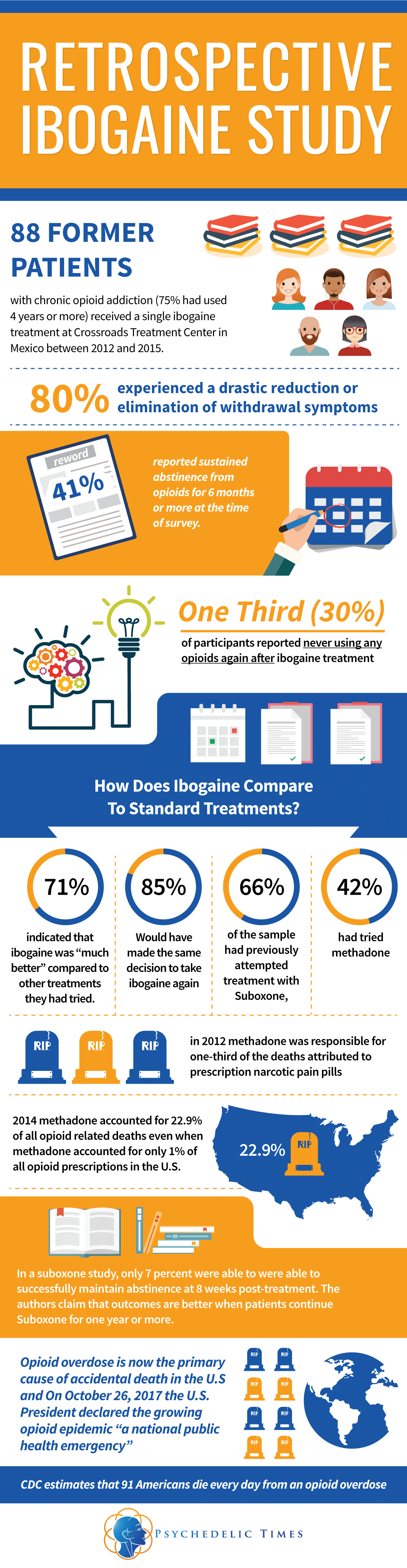
The largest observational study to date evaluating the efficacy of ibogaine treatment for opioid use disorder (OUD) was recently published in the peer-reviewed Journal of Psychedelic Studies. The retrospective study, published in September 2017, enrolled 88 former patients with chronic opioid use (75% had used 4 years or more) who had previously received ibogaine treatment at the Crossroads Treatment Center (Crossroads) in Mexico between 2012 and 2015. The collaborative study, conducted by members from the John Hopkins Behavioral Pharmacology Research Unit, Crossroads Treatment Center, and the Yale School of Medicine, shows promising evidence for ibogaine as a treatment for people struggling with opioid addiction.
In the retrospective ibogaine study, the majority of individuals (80%) experienced a drastic reduction or elimination of withdrawal symptoms, and 41 percent reported sustained abstinence from opioids at the time of survey, being opioid free for at least 6 months or more. The authors found that about one third (30%) of participants reported never using any opioids again after ibogaine treatment. In this one third that reported never using opioids again, at the time of follow up, 54% had maintained abstinence for at least 1 year and 31% had been abstinent for 2 or more years. Although 48% of the full sample reported a lapse or relapse after treatment, they reported decreased consumption from pretreatment levels.
The results of the study are very encouraging when compared to traditional opioid maintenance therapies (OMT) and considering the magnitude of the current national opioid crisis. 66% of the sample of ibogaine patients had previously attempted treatment with Suboxone, and 42% had tried methadone, the most common forms of OMT. In contrasting ibogaine to mainstream approaches to addressing addiction, 85% said that looking back they would have made the same decision to take ibogaine again and 71% indicated that ibogaine was “much better” compared to other treatments they had tried. The results of the study are particularly notable considering that on October 26, 2017 the U.S. President declared the growing opioid epidemic, “a national public health emergency.” The U.S. accounts for a quarter of drug-related deaths worldwide, and drug overdose, largely due to opioids, is now the primary cause of accidental death in the U.S. In the midst of the current opioid epidemic, Americans are struggling to find effective treatment options for OUD, and ibogaine advocates are not giving up in working to make this treatment available.
Legalization Efforts
In the past two years, three states have already introduced legislation for the medical use or scientific research of ibogaine. Vermonters for Ibogaine Research (VFIR), who introduced House Bill 387 and 741, testified before the House Committee on Human Services for the establishment of an ibogaine-assisted pilot program (3, 4). New York State Representative David Weprin introduced Assembly Bill A08356, which would require the start of clinical research into the use of ibogaine in drug treatment (5). In Maryland, State Delegate Wendell R. Beitzel sponsored House Bill 1372 (6), a bill that would establish an ibogaine treatment pilot program. Shortly after President Trump’s inauguration in January 2017, the New York Times carried a full page letter inviting the President to make the opioid epidemic and ibogaine as a potential treatment a crucial issue in his first 100 days of office.
Although the bills in New York, Vermont, and Maryland failed to pass, these efforts pave the way for future legislation to support ibogaine research and legalize treatment on the state level. Earlier this year, the Multidisciplinary Association for Psychedelic Studies (MAPS) proposed a comment for consideration to the President’s Commission on Combating Drug Addiction and the Opioid Crisis to fund clinical research on ibogaine as a uniquely effective treatment for opioid addiction (7). Despite having the same legal status as ibogaine, cannabis is currently legal for medical purposes in over half of the states in the U.S. Ibogaine is different from cannabis and many other Schedule 1 drugs as it has little to no history of recreational use or stigma, is non-addictive, has multiple potential medical uses, and is primarily sought for treating addiction or for spiritual/ceremonial purposes.
Ibogaine vs Conventional Treatments
The most commonly utilized intervention in the U.S. for opioid use disorders (OUD) are opioid maintenance therapies (OMT). The goal with OMT is often abstinence (8) and according to the National Institute on Drug Abuse, OMT may decrease opioid use, opioid-related deaths, criminal activity, and infectious disease transmission (9). While these medications may help, they are not risk-free and a high percentage of individuals often relapse or overdose shortly after tapering off of OMT or overdose directly on OMT (10). Additionally, OMT are problematic because they perpetuate physical and physiological dependence on opioids, requiring long-term use, sometimes for years, and ongoing monitoring for side effects (11). For example, a methadone prescription is recommended to be taken for a minimum of one year before tapering off (20).
OMT are frequently hazardous and their efficacy is less than ideal. Methadone is the most widely used OMT and is deemed safe and effective, yet in 2012 methadone was responsible for one-third of the deaths attributed to prescription narcotic pain pills (19). According to the CDC, in 2014 methadone accounted for 22.9% of all opioid related deaths even when methadone accounted for only 1% of all opioid prescriptions in the U.S. In a heavily cited review involving almost two thousand methadone patients, the treatment increased patient retention and reduced heroin use while patients were on methadone; ultimately, however, it did not affect mortality or criminal activity (12). In another OMT study using buprenorphine-naloxone (brand name Suboxone), at 8 weeks post-treatment only 7 percent of the study participants were able to successfully maintain abstinence. The authors of the Suboxone study claimed that OMT demonstrate better outcomes when patients continued to participate in treatment for a year or more (13). This response rate is much lower, and of a shorter duration compared to the current and prior ibogaine studies.
Safety concerns have been cited as a reason to keep ibogaine classified as a Schedule 1 substance in the U.S. A literature review covering medical records from about two decades, 1990 to 2008 shows that 19 people died from about an hour to three days after using ibogaine. None of the deaths however, were proven to be directly attributed to ibogaine, but rather to its interactions with prior medical conditions (primarily pre-existing heart conditions) or other drugs taken with the ibogaine (17). The deaths associated with ibogaine use pale in comparison to deaths attributed to methadone. Currently, the main barriers to researching the safety of ibogaine treatment are funding constraints and legality (16).
The CDC estimates that 91 Americans die everyday from an opioid overdose (14). This issue is an urgent crisis and patients and families do not have time to wait a year, which is the minimum time recommended for a methadone prescription. A safe and effective treatment for OUD should not take a year to be effective, nor should it be responsible for causing a large percentage of overdoses, which is the case with methadone. Unlike OMT drugs, ibogaine does not perpetuate dependency on opioids and it can rapidly alleviate withdrawal symptoms and cravings within hours. Despite the limitations of the current ibogaine study (such as the retrospective nature, self-selection bias, and self-report design), the broad efficacy rates for ibogaine from this study and others suggest massive potential for ibogaine to be an innovative, safe, and rapid option for addressing the opioid epidemic and lack of effective treatments.
A Need for Further Research and Action
There have not been any published controlled clinical trials to investigate ibogaine as a therapeutic medicine, and clinical data is limited to two open label case series, both of which show that a single dose of ibogaine significantly reduces opioid withdrawal symptoms in most patients (18). Ibogaine is not expected to be legalized in the U.S. any time soon, but considering the urgency of a suitable treatment for opioid misuse, the tides may change in the coming years. After learning of the benefits of ibogaine treatment, more and more individuals are seeking to raise awareness regarding the future of ibogaine research and treatment in the U.S. You can play a part too; below we have listed some ways you can participate and show your support:
- Donate or volunteer for organizations that support ibogaine clinical guidelines and research such as the Global Ibogaine Therapy Alliance and the Multidisciplinary Association for Psychedelic Studies
- Attend an event or a conference (conferences may offer funds for volunteering or presentations):
- Host an ibogaine fundraiser. Talk about it with your community, outlining its potential uses and benefits, and comparing its effectiveness with existing treatments. For more info on creating a successful event, check out the SSDP Access to Harm Reduction Campaign Toolkit.
- Contact ibogaine policy makers like those groups in New York, Vermont and Maryland who already proposed bills to continue to support the development of state level legalization.
- Write a blog post about ibogaine (this one is an example).
- Share this and other relevant information on ibogaine on social media.
References
- https://www.springer.com/us/book/9783319497112
- https://www.ncbi.nlm.nih.gov/pubmed/19834993
- https://legislature.vermont.gov/assets/Documents/2016/Docs/BILLS/H-0387/H-0387%20As%20Introduced.pdf
- https://legislature.vermont.gov/assets/Documents/2016/Docs/BILLS/H-0741/H-0741%20As%20Introduced.pdf
- https://assembly.state.ny.us/leg/?default_fld=&bn=A08356&term=2015&Summary=Y&Actions=Y&Memo=Y&Text=Y
- https://mgaleg.maryland.gov/2017RS/fnotes/bil_0002/hb1372.pdf
- https://s3-us-west-1.amazonaws.com/mapscontent/images/MAPS.org/OpioidCommission-MAPS-Comment-06.12.17.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/25747920
- https://www.drugabuse.gov/publications/effective-treatments-opioid-addiction/effective-treatments-opioid-addiction
- https://www.nejm.org/doi/full/10.1056/NEJMra1604339#t=article
- https://www.nejm.org/doi/full/10.1056/NEJMra1604339#t=article
- https://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002209.pub2/abstract
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3470422/
- https://www.cdc.gov/drugoverdose/epidemic/index.html
- https://www.cdc.gov/mmwr/volumes/66/wr/mm6612a2.htm?s_cid=mm6612a2_w
- https://www.ncbi.nlm.nih.gov/pubmed/23627782
- https://www.ncbi.nlm.nih.gov/pubmed/22268458
- https://www.ncbi.nlm.nih.gov/pubmed/18029124
- https://www.cbsnews.com/news/methadone-to-blame-for-one-third-of-us-prescription-painkiller-deaths-cdc-says/
- https://www.samhsa.gov/medication-assisted-treatment/treatment/methadone
About the Authors:
Lead Writer
Kevin Garcia is a member of the board of directors of Students for Sensible Drug Policy. He has hosted and spoken at events and conferences on post traumatic stress, problematic drug use, harm reduction, overdose prevention, psychedelic research, student activism, cognitive neuroscience, and medical marijuana. Mr. Garcia holds a Bachelors degree in Psychology from Florida International University.
Writer/ Editor:
Dr. Joseph Barsuglia is a research psychologist, clinical research director of Crossroads Treatment Center (which utilizes ibogaine and 5-MeO-DMT in the treatment of addiction), and a psychotherapist in the phase 3 clinical trials of MDMA-Assisted psychotherapy for PTSD sponsored by MAPS in Los Angeles. He is a member of the board of directors of Source Research Foundation and the Terra Incognita Project which are non-profit organizations dedicated to advancing science, research, and community on psychedelics and consciousness. He is also on the board of LightStar, a business igniter and holding company dedicated to scaling future clinical paradigms for psychedelic medicine.